Axsome Therapeutics (NASDAQ:AXSM) is increasingly poised to become the single most important player in the Major Depressive Disorder space, with its Auvelity tracking ahead of market expectations. In the last quarter, Auvelity sales were $27.6 million, representing 76% quarter-over-quarter growth. Being the first and only oral rapid acting NMDA receptor antagonist for MDD, Auvelity has had a tremendous Rx growth since its approval in October 2022, as we can see from the chart below:
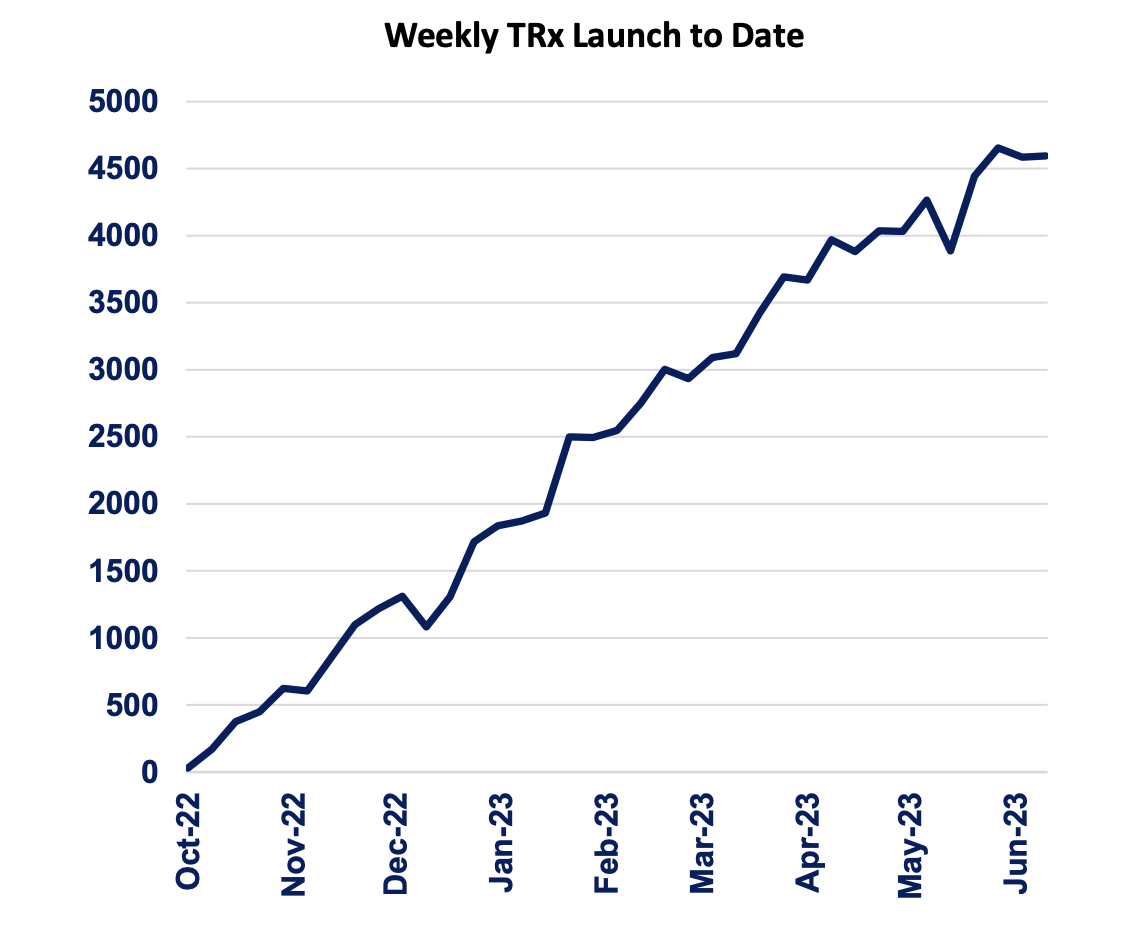
Auvelity weekly Trx (AXSM website)
One of the most striking features of this treatment is the rapid onset of symptom improvement, a remarkable departure from traditional oral antidepressants. While many antidepressant medications often require several weeks to begin showing any discernible effects, Auvelity starts producing noticeable benefits as early as Week 1 of treatment, presenting a compelling solution for patients seeking swift relief. What’s even more remarkable is that this relief is not fleeting; it is sustained and becomes increasingly effective as time progresses, continuing through to Week 6. Auvelity shows remission as early as Week 2, unlike the drawn out remission of many standard MDD drugs. It is not easy to bring a new drug to the market and change the treatment paradigm; but Axsome has been able to do just that with Auvelity in MDD.
A key paragraph from BofA analysts discussing some of the selling points of Auvelity in MDD includes the following interesting excerpt:
BofA also said it believes Auvelity can hang on to its market exclusivity until 2034. Also brightening the drug’s prospects is the recent failure by Sage Therapeutics (SAGE) and Biogen (BIIB) to win FDA approval for their MDD drug zuranolone. As a result, the bank has upped its peak Auvelity MDD sales projections to $1.2B from $830M.
The sales team for Auvelity has been increased from 160 to 260, improving their reach from “26,000 to approximately 44,000 physicians who write greater than 80% of branded antidepressant prescriptions.” Payor coverage has improved vastly, now covering 68% of all covered lives.
A label expansion for Auvelity in Alzheimer’s agitation is in the works. The drug comes with a breakthrough therapy designation in this indication, so it will likely get privileged treatment in the form of quicker review time. Unlike the risky area of Alzheimer’s Disease, this drug does not target the disease itself, but just a byproduct. The primary endpoints were successfully achieved in two rigorously conducted clinical trials. In the ADVANCE-1 Phase 2/3 trial, which featured parallel group design, the objectives were met. The ACCORD Phase 3 trial, a randomized withdrawal trial, also achieved its primary endpoints. Additionally, there is an ongoing ADVANCE-2 Phase 3 trial, with an anticipated completion date in the first half of 2024. A likely approval sometime in 2025 is possible here. In the ADVANCE-1 trial, the drug achieved statistical significance at week 5 versus both bupropion and placebo in CMAI total score.
Another emerging gamechanger for the company is sunosi, which they acquired from Jazz Pharmaceuticals in a surprisingly lenient deal. This drug is approved for Excessive Daytime Sleepiness (EDS’) Associated with Narcolepsy or Obstructive Sleep Apnea (OSA), and last quarter made $19.1mn in net product revenue. This is more than double the figure we saw in the comparable period last year.
AXS-07 in migraine and AXS-14 in fibromyalgia are two other late stage product candidates. AXS-14 has previously met the primary endpoints and demonstrated positive and statistically significant results in a Phase 3 and in a Phase 2 trial for the management of fibromyalgia. An NDA submission is planned for Q4 2023. AXS-12 topline results in narcolepsy are also slated to be out in the third quarter of 2023. Another lead catalyst right now should be the planned resubmission of the NDA for AXS-07 for migraine. The FDA required certain manufacturing issues to be sorted out, which is now work in progress, and should be completed in the first half of 2024. No new safety or efficacy data has been requested.
All in all, AXSM, despite the approval, is in a catalyst-rich environment today. Here’s a list of anticipated milestones for the next year:
Regulatory and Commercial:
-
AXS-07 for migraine, NDA resubmission (1H 2024)
-
AXS-14 for fibromyalgia, NDA submission (4Q 2023 – 1Q 2024)
Clinical Trial Readouts:
-
Phase 3 SYMPHONY trial of AXS-12 in narcolepsy (4Q 2023)
-
Phase 3 ADVANCE-2 trial of AXS-05 for Alzheimer’s disease agitation (1H 2024)
-
Phase 3 FOCUS trial of solriamfetol in ADHD in adults (2H 2024)
Clinical Trial Initiations:
-
Phase 3 trial of solriamfetol for binge eating disorder (4Q 2023)
-
Phase 3 trial of solriamfetol in shift work disorder (1Q 2024)
-
Pivotal Phase 2/3 trial of AXS-05 for smoking cessation (4Q 2023 – 1Q 2024)
Financials
AXSM has a market cap of $3.14bn and a cash balance of $468mn (pro forma) as of June 2023. Research and development (R&D) expenses were $20.6 million for the second quarter of 2023, cost of revenue was $4.6 million, and Selling, general, and administrative (SG&A) expenses were $78.9 million. The company has a surprisingly low R&D expense figure given its multiple late stage trials; new trial initiations may increase this figure. The company anticipates that this cash will be enough to last them until cash flow positivity.
Bottomline
I have been in AXSM for 3 years, and my usual strategy is to exit emerging companies as they “emerge,” because long experience has taught me that the first approval is often followed by an anticlimactic price movement. So, in biopharma generally, the best time to be out is before they actually prove themselves in the market – because often, they don’t. That’s a cynical attitude, admittedly, however sometimes, a rare company will make an exception to that rule. AXSM is one such rare company. I made profits on their first approval, however, I am convinced that given their lineup of catalysts and milestones, strong sales, flawless trial executions, and so on, AXSM is a really long-term buy-the-dip and-hold.
About the TPT service
Thanks for reading. At the Total Pharma Tracker, we offer the following:-

Our Android app and website feature a set of tools for DIY investors, including a work-in-progress software where you can enter any ticker and get extensive curated research material.
For investors requiring hands-on support, our in-house experts go through our tools and find the best investible stocks, complete with buy/sell strategies and alerts.
Sign up now for our free trial, request access to our tools, and find out, at no cost to you, what we can do for you.

Read the full article here











Leave a Reply